Understanding the Gut Brain Axis & Systemic Stress
The intricate relationship between our gut and brain, known as the gut-brain axis, has garnered significant attention in recent years, particularly...
2 min read
 Manoj Dadlani
:
Apr 3, 2025 10:15:29 AM
Manoj Dadlani
:
Apr 3, 2025 10:15:29 AM
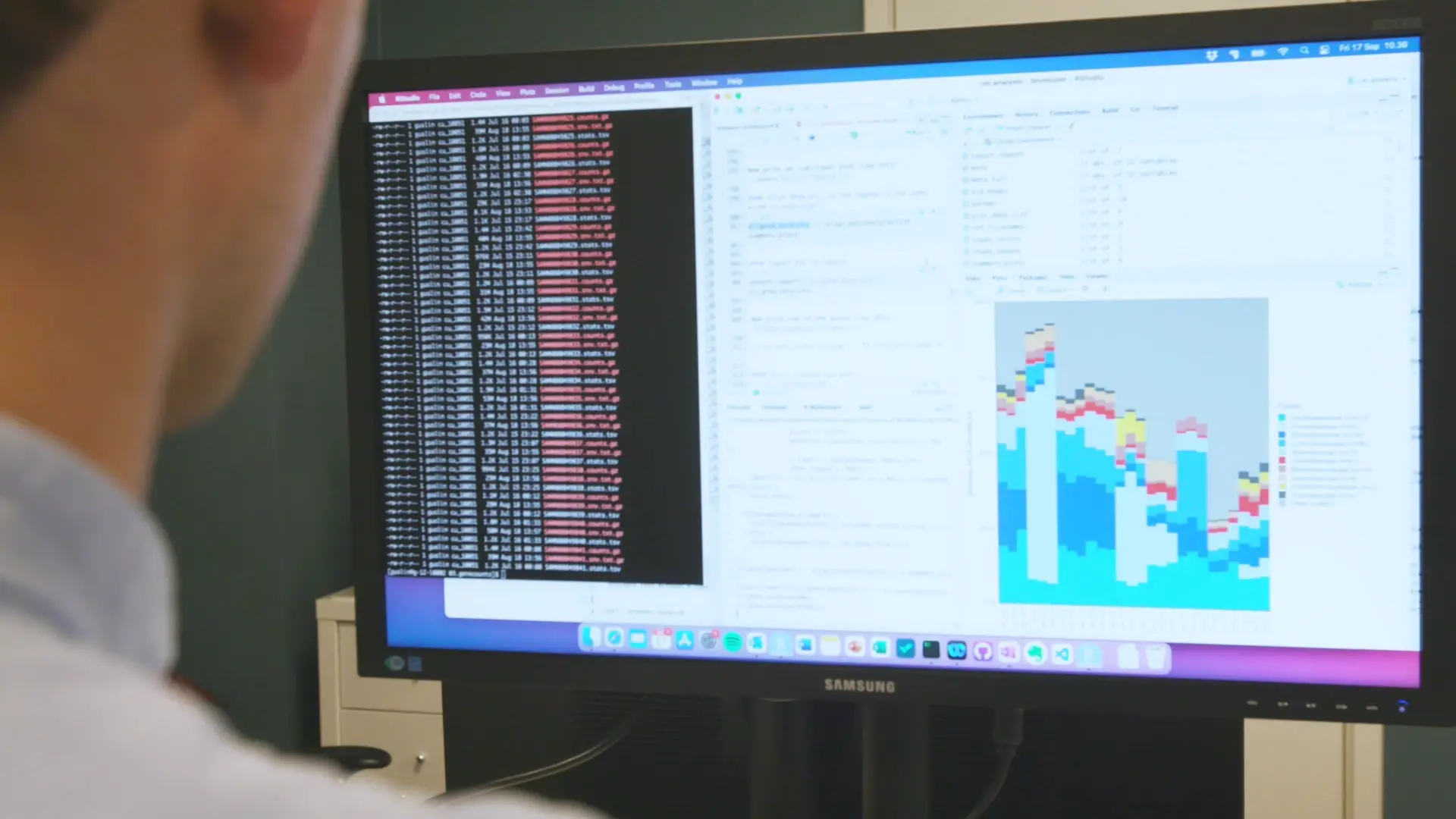
Grabrucker S, Marizzoni M, Silajdžić E, et al. Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain. 2023;146:4916-4934. https://doi.org/10.1093/brain/awad303
Our metabolomics capabilities were recently highlighted in an Alzheimer’s Fecal Microbiota Transplant (FMT) study published in BRAIN by researchers at University College Cork in Ireland. By utilizing untargeted metabolomics, Grabrucker, Marizzoni, and colleagues underpinned novel microbiome-mediated mechanisms that induce Alzheimer’s symptoms in healthy animals upon transfer of diseased-animal gut contents. This data supports the systemic role of gut microbiota in Alzheimer’s disease progression, specifically in cognitive decline and altered hippocampal neurogenesis.

Leveraging our capabilities in liquid chromatography-mass spectrometry (LC-MS), our team identified 245 metabolites from our internal database in caecal content and hippocampal tissue of young adult rats. This data was pivotal in identifying metabolic alterations following the transplantation of gut microbiota from Alzheimer’s models. In addition, we leveraged external databases to infer several hundred additional metabolites, enriching the dataset and expanding the scope of our metabolic analysis.
The metabolomic profiles revealed through our analysis highlighted distinct differences between Alzheimer’s-affected and healthy control animals. Notably, we identified significant alterations in key metabolites, including:
Neuroactive Amino Acids:
– Histidine and derivatives (increased in AD ceca) – involved in the regulation of systemic inflammation and neural signaling that may influence neurotoxicity of Aβ fibrils.
– Taurine (decreased in AD hippocampus) – known to regulate adult hippocampal neurogenesis and associated with cognitive function.
– Aminoadipic acid (increased in AD ceca) – linked to neurodegenerative disease progression and could be a potential biomarker for early diagnosis or a target for therapeutic intervention.
– Homocitrulline (decreased in AD hippocampus) – alterations in citrullination have been linked to early Alzheimer’s disease pathology.
– 4-Guanidinobutyric Acid (decreased in AD hippocampus) – alterations in this metabolite have been observed in Alzheimer’s disease models.
Organic Acids:
– Galactonic acid (decreased in AD ceca) – correlated with gut microbiota changes in AD models.
– Succinic acids (increased in AD ceca) – integral to cellular metabolism and may reflect underlying mitochondrial dysfunctions associated with AD.
– Tetradecanedioic acid (increased in AD ceca) – linked to aging and prediction of neurocognitive disorders.
Tryptophan Metabolites:
– Kynurenic acid (increased in AD ceca) – possesses neuroprotective properties and may play a role in modulating glutamatergic neurotransmission.
– Xanthurenic acid (increased in AD ceca) – previously implicated in neurological disorders
– Overall disruption in tryptophan metabolism is known to influence brain function and neurodegenerative diseases like Alzheimer’s.
Ketone Bodies
– Hydroxybutyrate (decreased in AD ceca) – known to mitigate Alzheimer’s disease pathogenesis through various mechanisms.
These metabolites are intricately linked with various biological processes that could potentially influence the onset and progression of Alzheimer’s disease, offering new targets for therapeutic intervention and a deeper understanding of disease mechanisms.
While our laboratory focused on the generation of untargeted metabolomic data, the principal investigators undertook an extensive multi-omics analysis, integrating our findings with additional omic layers. This multidisciplinary approach enhances the study’s depth and facilitates a holistic understanding of Alzheimer’s Disease.
This study not only emphasizes the critical role of the microbiota-gut-brain axis in Alzheimer’s disease but also highlights our capability to deliver high-quality metabolomics data that can drive significant advancements in neurodegenerative disease research.
Understanding how specific gut microbiota-induced metabolic changes correlate with Alzheimer’s pathology enables the identification of early intervention points that could delay or potentially reverse disease progression.
We are proud to contribute to this important work and excited about the potential implications of our findings for developing novel diagnostics and treatments. As we continue to explore the vast possibilities of metabolomics, we remain committed to supporting the scientific community in its quest to unravel the mysteries of Alzheimer’s disease and beyond.
Stay connected for more updates as we further our mission to unlock the microbiome through precision metabolomics.
The intricate relationship between our gut and brain, known as the gut-brain axis, has garnered significant attention in recent years, particularly...
.webp)
The gut microbiome, an intricate ecosystem of trillions of microorganisms living throughout the human gastrointestinal tract, plays a crucial role in...

In this exploration, we delve into the fascinating connection between Irritable Bowel Syndrome (IBS), Inflammatory Bowel Disease (IBD), and the...